Insights
Research Story Tip: A First Look at How the Drug Salvinorin A Works in the Brain
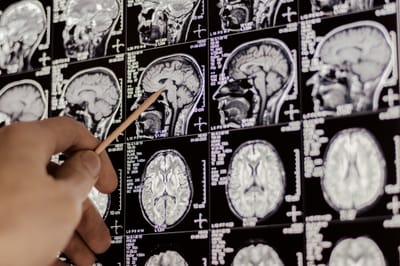
Three different fMRI scans of the brain from a recent Johns Hopkins Medicine study show that the psychedelic salvinorin A (derived from the Salvia divinorum plant seen in the background) affects the default mode network (seen in red) — the area of the brain most active when a person is not mentally engaged. Credit: Graphic created by M.E. Newman, Johns Hopkins Medicine, using public domain image of Salvia plant and fMRI images courtesy of Manoj Doss, Johns Hopkins Center for Psychedelic and Consciousness Research
Read More
The Acute Effects of the Atypical Dissociative Hallucinogen Salvinorin A on Functional Connectivity in the Human Brain
Salvinorin A (SA) is a κ-opioid receptor agonist and atypical dissociative hallucinogen found in Salvia divinorum. Despite the resurgence of hallucinogen studies, the effects of κ-opioid agonists on human brain function are not well-understood. This placebo-controlled, within-subject study used functional magnetic resonance imaging for the first time to explore the effects of inhaled SA on strength, variability, and entropy of functional connectivity (static, dynamic, and entropic functional connectivity, respectively, or sFC, dFC, and eFC). SA tended to decrease within-network sFC but increase between-network sFC, with the most prominent effect being attenuation of the default mode network (DMN) during the first half of a 20-min scan (i.e., during peak effects). SA reduced brainwide dFC but increased brainwide eFC, though only the former effect survived multiple comparison corrections. Finally, using connectome-based classification, most models trained on dFC network interactions could accurately classify the first half of SA scans. In contrast, few models trained on within- or between-network sFC and eFC performed above chance. Notably, models trained on within-DMN sFC and eFC performed better than models trained on other network interactions. This pattern of SA effects on human brain function is strikingly similar to that of other hallucinogens, necessitating studies of direct comparisons.
Read More
Solving an Old Puzzle: Elucidation and Evaluation of the Binding Mode of Salvinorin A at the Kappa Opioid Receptor
The natural product Salvinorin A (SalA) was the first nitrogen-lacking agonist discovered for the opioid receptors and exhibits high selectivity for the kappa opioid receptor (KOR) turning SalA into a promising analgesic to overcome the current opioid crisis. Since SalA’s suffers from poor pharmacokinetic properties, particularly the absence of gastrointestinal bioavailability, fast metabolic inactivation, and subsequent short duration of action, the rational design of new tailored analogs with improved clinical usability is highly desired. Despite being known for decades, the binding mode of SalA within the KOR remains elusive as several conflicting binding modes of SalA were proposed hindering the rational design of new analgesics. In this study, we rationally determined the binding mode of SalA to the active state KOR by in silico experiments (docking, molecular dynamics simulations, dynophores) in the context of all available mutagenesis studies and structure-activity relationship (SAR) data. To the best of our knowledge, this is the first comprehensive evaluation of SalA’s binding mode since the determination of the active state KOR crystal structure. SalA binds above the morphinan binding site with its furan pointing toward the intracellular core while the C2-acetoxy group is oriented toward the extracellular loop 2 (ECL2). SalA is solely stabilized within the binding pocket by hydrogen bonds (C210ECL2, Y3127.35, Y3137.36) and hydrophobic contacts (V1182.63, I1393.33, I2946.55, I3167.39). With the disruption of this interaction pattern or the establishment of additional interactions within the binding site, we were able to rationalize the experimental data for selected analogs. We surmise the C2-substituent interactions as important for SalA and its analogs to be experimentally active, albeit with moderate frequency within MD simulations of SalA. We further identified the non-conserved residues 2.63, 7.35, and 7.36 responsible for the KOR subtype selectivity of SalA. We are confident that the elucidation of the SalA binding mode will promote the understanding of KOR activation and facilitate the development of novel analgesics that are urgently needed.
Read More